A
battery is a device that can store energy in a chemical form and
convert that stored chemical energy into electrical energy when
needed. There are no batteries that actually store electrical
energy; all batteries store energy in some other form. There are two
fundamental types of chemical storage batteries: the rechargeable, or
secondary cell, and the non-rechargeable, or primary cell. In terms
of storing energy or discharging electricity, they are similar, it
is simply a question of whether or not the chemical processes
involved permit multiple charging and discharging. The
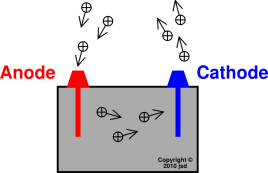
chemical reaction at the anode releases electrons and the reaction at
the cathode absorbs them. When the electrical path provided by the
electrolyte and an external electrical circuit connects the anode and
cathode, the two simultaneous reactions proceed and the electrons
freed at the anode travel through the external electrical connection
and react chemically at the cathode to make the cell function.
A
cathode
is
the electrode from which a conventional current leaves a polarized
electrical device. An
anode
is
an electrode through which electric current flows into a polarized
electrical device. An
electrolyte
is a substance that ionizes
when
dissolved in suitable ionizing solvents.
When
electrodes
are
placed in an electrolyte and a voltage
is
applied, the electrolyte will conduct electricity. Lone electrons
normally
cannot pass through the electrolyte; instead, a chemical reaction
occurs at the cathode,
consuming electrons from the anode.
Lead
acid batteries
Manufacturers
of lead acid batteries
EnerSys operates over twenty manufacturing and assembly plants in Europe, North America and Asia. EnerSys also provides aftermarket and customer support services to its customers from over 100 countries through its sales and manufacturing locations around the world.
South
Africa
- First National Battery is the leading lead acid battery manufacturer in South Africa, producing over 2.2 million batteries a year. Their batteries are used in more than 40 countries and the products cover various industries and applications ranging from mining, railway and renewable energy to surface traction, telecommunications and automotive (including industrial, commercial and passenger vehicles). First National Battery manufactures and distributes the industry-leading Raylite and Exide lead acid batteries. Batteries produced by First National Battery are the first choice among South African Original Equipment Manufacturers (OEMs) including Mercedes Benz, Toyota, Nissan, GM SA, BMW, Volkswagen SA, Renault, Ford, Nissan Diesel and MAN.
Dry
cell batteries

1. Brass cap
2.
Plastic seal
3.
Expansion space
4.
Porous cardboard
5.
Zinc can
6.
Carbon rod
7.
Chemical
mixture.
A
standard dry cell comprises a zinc
anode,
usually in the form of a cylindrical pot,
with a carbon
cathode
in
the form of a central rod. The electrolyte
is
ammonium
chloride
in
the form of a paste next to the zinc anode. The remaining space
between
the electrolyte and carbon cathode is taken up by a second paste
consisting of ammonium
chloride
and
manganese
dioxide,
the latter acting as a depolariser.
In some designs, the ammonium chloride is replaced by zinc
chloride.
Alkaline
batteries are
dependent upon the reaction between zinc
and
manganese
dioxide.
A rechargeable
alkaline battery
allows
reuse of specially designed cells. Compared with zinc-carbon
batteries
of
the
zinc chloride types, alkaline batteries have a higher energy
density
and
longer shelf-life,
with the same voltage. Button
cell
silver-oxide
batteries
have
higher energy density and capacity but also have a higher cost than
similar-size alkaline cells.
The
alkaline battery
gets its name because it has an alkaline
electrolyte
of potassium
hydroxide,
instead of the acidic ammonium
chloride
or
zinc
chloride
–manganese
dioxide
combination).A
dry cell uses a paste electrolyte,
with only enough moisture
to
allow current to flow. Unlike a wet
cell,
a dry cell can operate in any orientation without spilling, as it
contains no free liquid.
A
common dry cell is the zinc–carbon
battery
with
a nominal voltage of 1.5 volts,
the same as the alkaline
battery
(since
both use the same zinc
electrolyte
of the zinc-carbon batteries. Other battery systems also use alkaline
electrolytes, but they use different active materials for the
electrodes.
Lithium
batteries are
batteries
that
have lithium
metal
or lithium compounds as a anode.
They stand apart from other batteries in their high charge density
(long life) and high cost per unit. Depending on the design and
chemical compounds used, lithium cells can produce voltages from 1.5
V (comparable to a zinc–carbon
or
alkaline
battery)
to about 3.7 V. By comparison,
lithium-ion
batteries
are
rechargeable
batteries
in
which lithium ions move between the anode and the cathode, using
an
intercalated
lithium
compound
as
the electrode
material
instead of the metallic lithium used in lithium batteries.
South
Africa
- Eveready produces alkaline, lithium, zinc round cells , zinc layer cells and rechargeable cells under the brand names Eveready and Ecocell .
Lithium-ion
battery
A Lithium-ion battery (sometimes Li-ion battery or LIB) is a member of a family of rechargeable
battery
types
in which lithium
ions
move from the negative electrode to the positive electrode during
discharge and back when charging. Handheld
electronics mostly use LIBs based on lithium
cobalt oxide
(LiCoO2),
which offers high energy density, but presents safety risks,
especially when damaged.
Lithium
iron phosphate
(LFP),
lithium
manganese oxide
(LMO)
and
lithium nickel
manganese cobalt oxide (NMC) offer
lower energy density, but longer lives and inherent safety. According
to Frost & Sullivan, a leading growth-consulting firm, the global
market of rechargeable lithium-ion batteries is projected to be worth
US$23.4 billion in 2016.
- In June 2015, 24M Technologies Inc claimed that they will reduce Li-ion battery costs to 50% of today’s costs with their semi-solid lithium ion battery and that it could also fundamentally change the entire cost structure of the industry. Lithium-ion battery cells typically use graphite for the anode. Manufacturers have looked into the benefit of using silicon for the anode because silicon can hold a lot more lithium ions.
- Researchers at MIT and Samsung, and in California and Maryland, have developed a new approach to one of the three basic components of batteries, the electrolyte. The new findings are based on the idea that a solid electrolyte, rather than the liquid used in today’s most common rechargeables, could greatly improve both device lifetime and safety — while providing a significant boost in the amount of power stored in a given space.
- Scientists at Nanyang Technology University (NTU) have developed ultra-fast charging batteries that can be recharged up to 70 per cent in only two minutes. The new generation batteries also have a long lifespan of over 20 years, more than 10 times compared to existing lithium-ion batteries. In the new NTU-developed battery, the traditional graphite used for the anode (negative pole) in lithium-ion batteries is replaced with a new gel material made from titanium dioxide.
Manufacturers
of lithium-ion batteries
A123
Systems, LLC develops and manufactures advanced
Nanophosphate® lithium
iron phosphate batteries ( at the cell, module and system level,)
and energy storage systems (ESS). A123 Systems' head
quarter is in Livonia, Michigan and employs more than 2,000 people
worldwide. A123 has more than 1 million square feet of manufacturing
facilities in Asia, Europe and North America.
Altairnano
is the first company to replace traditional graphite materials used
in conventional lithium-ion batteries with a proprietary,
nanostructured lithium-titanate. PowerRack is a complete industrial
battery system for commercial use and fully scalable to 40 modules
Johnson
Matthey Battery Systems (formerly Axeon) is Europe’s largest
independent Lithium-ion battery systems supplier, processing over 70
million cells a year and supplying volume production of batteries for
global markets. The
UK
operation focuses on the design and manufacture of large-scale high
voltage automotive grade battery systems for Electric and Hybrid
Electric vehicles and batteries for mobile power products. In Poland
they
design
and manufacture high performance battery packs for the professional
cordless power tools and electric bike markets.
BYD
Company Limited (BYD) is a listed company on Hong Kong Stock Exchange
and
Shenzhen
Stock Exchange, specialized in IT, automobile and new energy
industries. BYD has nearly 180,000 employees and 12 industrial parks
located in Shenzhen, Beijing, Xian, Shanghai, Changsha and other
cities across China with an area over 15,000,000 square meters. BYD
has formed a global network including branches or offices in United
States, Europe, Japan, India, Hong Kong, Taiwan, etc.
Electrovaya
designs, develops and manufactures proprietary Lithium Ion
SuperPolymer® 2.0 batteries, battery systems, and battery-related
products for the transportation, grid power, consumer and
healthcare markets.
LG
Chem produces cathode materials, electrolytes, and separators which
are core materials of rechargeable batteries. In addition to mobile
batteries used in various IT devices, they are also producing mid to
large battery materials for electric cars and energy storage systems.
Mitsubishi
Heavy Industries, Ltd. (MHI) has concluded an agreement with Delta
Electronics, Inc., a leading manufacturer of electronic devices
in Taiwan, under which MHI will sell Delta its business assets,
including machinery, in lithium-ion rechargeable batteries. As a
result MHI will shift its management resources into operations in
energy storage system (ESS) products employing lithium-ion
rechargeable batteries.
Development,
manufacturing and sales of NiMH batteries for PEV/HEV; lithium-ion
batteries and BMS (Battery Management System)
Powerwall
comes in 10 kWh weekly cycle and 7 kWh daily cycle
models. Both are guaranteed for ten years and are sufficient to
power most homes during peak evening hours. Multiple batteries may be
installed together for homes with greater energy need, up to 90 kWh
total for the 10 kWh battery and 63 kWh total for the 7 kWh battery.
Tesla's
Gigafactory will eventually have an annual production capacity
of 35,000,000 kWh, or 35 GWh.
For
home use a 2.5 kWh capacity unit with a maximum of 8 modules. They
also manufacture units for commercial and industrial companies.
Produces
a semi-solid
lithium-ion battery that
speeds
up
production
and reduces lithium-ion battery costs by 50%.
- StoreDot
StoreDot's FlashBattery technology relies on "nanodots", which are comprised of bio-organic peptides whose raw materials are abundant in nature and also self-assemble, making for a more affordable product. These form the basis of a multi-function electrode that allows for supercapacitor-like rapid charging, with a slow discharge more like a lithium-ion battery. The concept still includes lithium components in the cathode, but the company claims that its modified anode and cathode and a proprietary electrolyte and separator are responsible for the incredible recharge speeds.
Graphene and batteries
Graphene can make batteries that are light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery’s life-time, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries. Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries can be enhanced by introducing graphene to the battery’s anode and capitalizing on the material’s conductivity and large surface area traits to achieve morphological optimization and performance.
Commercial Graphene-enhanced battery products
Electric
vehicle batteries
An
electric
vehicle battery (EVB)
or traction
battery is
a battery used to power the propulsion of a battery
electric vehicles
(BEVs).
Rechargeable
batteries are usually the most expensive component of BEVs, being
about half the retail cost of the car. Since the late 1990s, advances
in battery technologies have been driven by demand for laptop
computers and mobile phones, with consumer demand for more features,
larger, brighter displays, and longer battery time driving research
and development
in
the field. The BEV marketplace has reaped the benefits of these
advances, but costs remain too high and, along with limited range,
provide a key barrier to the use of rechargeable batteries in
electric vehicles. The cost of electric vehicle batteries has been
reduced by more than 35% since 2008. Rechargeable traction batteries
are routinely used all day, and fast–charged all night. Forklifts,
for instance, are usually discharged and recharged every 24 hours of
the work week. The predicted market for automobile traction batteries
is over $37 billion in 2020.
On
an energy basis, the price of electricity to run an EV is a small
fraction of the cost of liquid fuel needed to produce an equivalent
amount of energy (energy
efficiency).
The cost of replacing the batteries dominates the operating costs.
Vehicle
|
Battery
|
Chevy
Volt
|
16.5
kWh
|
Nissan
Leaf
|
24
kWh
|
Chevy
Bolt
|
60
kWh
|
Tesla
Model S
|
70
kWh / 85 kWh
|
Liquid metal batteries
Initially based on magnesium and antimony as the negative and positive electrodes, respectively, and a low cost molten salt electrolyte, but transitioned to using a higher voltage and lower cost chemistry. All three active components are in liquid form when the battery operates. The two liquid electrodes are separated by a molten salt electrolyte, and these liquid layers float on top of each other based on density differences and immiscibility. The system operates at elevated temperature maintained by self-heating during charging and discharging. The result is a low-cost and long lifespan storage system.
Vanadium redox Flow Batteries
Connventional
technologies for the chemical storage of electricity (e.g.
electrolyzer, methanation, fuel cells) show a relatively low
efficiency. Redox Flow Batteries (RFB), however, show a DC-efficiency
of up to 80% – depending on the system and consideration – which
is within the range of efficiencies of conventional secondary
batteries (lithium-ion, lead or nickel-metal-hydride batteries). In
contrast to conventional battery systems in which power and storage
capacity are interrelated, Redox Flow Batteries have the ability to
scale power and storage capacity independently of each other.
Basically the RFB can cover a storage requirement of several hours to
a few days.
Capacitor
storage
A
capacitor
(originally
known as a condenser)
is a passive
two-terminal
electrical
component
used
to store energy
electrostatically
in
an electric
field.
The forms of practical capacitors vary widely, but all contain at
least two electrical
conductors
(plates)
separated by a dielectric
(i.e.
insulator).
A
capacitor
can store electric energy when disconnected from its charging
circuit, so it can be used like a temporary
battery,
or like other types of rechargeable
energy storage system.
Capacitors
are commonly used in electronic devices to maintain power supply
while batteries are being changed. Conventional
capacitors provide less than 360 joules
per
kilogram of energy
density,
whereas a conventional alkaline
battery
has
a density of 590 kJ/kg. In car
audio
systems,
large capacitors store energy for the amplifier
to
use on demand.
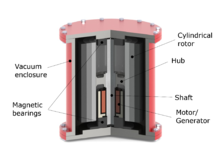
Flywheel energy storage
Flywheel
energy storage (FES)
works
by accelerating a rotor (flywheel)
to a very high speed and maintaining the energy in the system as
rotational
energy.
When energy is extracted from the system, the flywheel's rotational
speed is reduced as a consequence of the principle of conservation
of energy;
adding energy to the system correspondingly results in an increase in
the speed of the flywheel. Most
FES systems use electricity to accelerate and decelerate the
flywheel, but devices that directly use mechanical
energy
are
being developed. Since FES can be used to absorb or release
electrical energy such devices may sometimes be incorrectly and
confusingly described as either mechanical
or
inertia
batteries.
Advanced
FES systems have rotors made of high strength carbon fiber
composites, suspended by magnetic
bearings,
and spinning at speeds from 20,000 to over 50,000 rpm in a vacuum
enclosure.
Such
flywheels can come up to speed in a matter of minutes – reaching
their energy capacity much more quickly than some other forms of
storage.
Uninterruptible
power supply
An
uninterruptible
power supply,
also uninterruptible
power source,
(UPS)
or
battery/flywheel
backup,
is an electrical apparatus that provides emergency power to a load
when the input power source, typically mains
power,
fails. A UPS differs from an auxiliary or emergency
power system
or
standby
generator
in
that it will provide near-instantaneous protection from input power
interruptions, by supplying energy stored in batteries,
supercapacitors,
or flywheels.
The on-battery runtime of most uninterruptible power sources is
relatively short (only a few minutes) but sufficient to start a
standby power source or properly shut down the protected equipment.
An
UPS is typically used to protect hardware such as computers,
data
centers,
telecommunication
equipment
or other electrical equipment where an unexpected power disruption
could cause injuries, fatalities, serious business disruption or data
loss. UPS units range in size from units designed to protect a single
computer
without
a video monitor (around 200 volt-ampere
rating)
to large units powering entire data centers or buildings. The world's
largest UPS, the 46-megawatt Battery Electric Storage System (BESS),
in Fairbanks,
Alaska,
powers the entire city a
Using nature to grow batteries
Using nature to grow batteries

.png)






No comments:
Post a Comment